Product Applications
Hover and click on application to learn more ....
Previous
Next

Our Mission:
Help scientist with high quality in vivo imaging and preclinical research tools.

MediLumine offers a wide range of lab accessories and devices to help with in vivo research challenges.






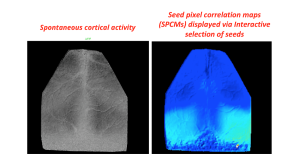
In their excellent review article titled ‘Not so spontaneous: Multi-dimensional representations of behaviors and context in sensory areas’ (ref. https://www.cell.com/neuron/pdf/S0896-6273(22)00588-8.pdf), Avitan and Stringer propose that

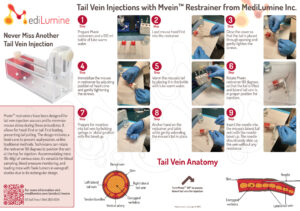
Wallchart Dimensions Width: 29.75″ – Height: 20.75″ Digital Version Download as PDF Working with tail vein injections in the lab? Request a free copy of

In their excellent review article titled ‘Not so spontaneous: Multi-dimensional representations of behaviors and context in sensory areas’ (ref. https://www.cell.com/neuron/pdf/S0896-6273(22)00588-8.pdf), Avitan and Stringer propose that

Wallchart Dimensions Width: 29.75″ – Height: 20.75″ Digital Version Download as PDF Working with tail vein injections in the lab? Request a free copy of

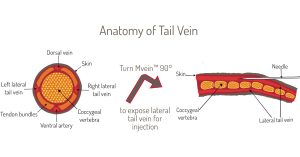
Achieving successful tail vein injections is no easy task. As seen in image below, the veins are small and there is a lot of room
Copyright © 2024 · MediLumine, Inc · All Rights Reserved